subcutaneous
administration

One monthly* dose.1
Flexible administration.1
HyQvia delivers long-lasting,† monthly* protection for your patients with PI who want a little more time between infusions.

*Every 3 or 4 weeks.
Median infusion time was 2.08 (0.83-4.68) hours vs 2.33 (0.92-6.33) for IVIG in the clinical trial.
†Between infusions.
With HyQvia, monthly* SCIG dosing could mean fewer infusions for your patients1
Number of infusions per year
50%
Patients’ number of infusions per year could be reduced by 50% or more
*Every 3 or 4 weeks.
cSCIG=conventional subcutaneous immune globulin.
Hyaluronidase is dosed at a fixed ratio of 0.5 mL rHuPH20 solution per 10 mL Immune Globulin Infusion 10% (Human) solution. This is supplied as part of a dual unit vial. Administer the full content of the Hy vial/s for both a full and partial dose of IG.
HyQvia dosing information
HyQvia [Immune Globulin Infusion 10% (Human) with Recombinant Human Hyaluronidase] comes in a dual vial unit—one vial of Immune Globulin Infusion 10% (Human) Solution [IG 10%] and one vial of Recombinant Human Hyaluronidase.1
The HyQvia dose is based on the immune globulin (IG 10%) component, which provides the therapeutic effect. Dispersion and absorption of the 10% IG is facilitated by the Recombinant Human Hyaluronidase.1
HyQvia initial monthly* dosing (following ramp-up) may vary depending on whether patients are switching from intravenous immune globulin (IVIG) treatment or conventional subcutaneous immune globulin (SCIG) treatment.1
*Every 3 or 4 weeks.
HyQvia dosing calculator
Determine dosage range
To determine the dosage ranges for a patient, select the weight closest to the patient’s weight in the window on the left. Dosage ranges will appear in grams. See Full Prescribing Information for additional dosage recommendations. A conversion factor of 2.2046 lb/kg is used to convert the patient’s weight to kilograms. The values displayed are rounded to the nearest decimal point.
What is the patient's weight?
Dosage range to be prescribed every 3-4 weeks or per patient needs
This calculator does not represent every possible weight calculation. Weights begin as 24 lb and increase in 4 lb increments to 288 lb.
Select available HyQvia vial combinations based on dosage
When available HyQvia solution vial sizes cannot be combined to equal the calculated IG dose, the dose must be rounded up or down.
The Physicians should make this determination based on their clinical judgment and HyQvia vial sizes.
HyQvia doses, based on available vial combinations, are shown in the table below.
| 2.5 | 5 | 7.5 | 10 |
| 12.5 | 15 | 17.5 | 20 |
| 22.5 | 25 | 27.5 | 30 |
| 32.5 | 35 | 37.5 | 40 |
| 42.5 | 45 | 47.5 | 50 |
| 52.5 | 55 | 57.5 | 60 |
| 62.5 | 65 | 67.5 | 70 |
| 72.5 | 75 | 77.5 | 80 |
*HyQvia is available in 2.5, 5, 10, 20, and 30 g vials of IG 10%. This table shows possible vial combinations at increasing 2.5 g increments, reading from left to right and top to bottom.
Switching patients to HyQvia
When switching to HyQvia, dose and frequency are determined by whether a patient is switching from an IVIG or a SCIG.1
Patients switching from IVIG treatment
Same dose and frequency of IVG
(after initial ramp-up)
Patients switching from SCIG
300 to 600 mg/kg at 3- or 4-week intervals
(after initial ramp-up)
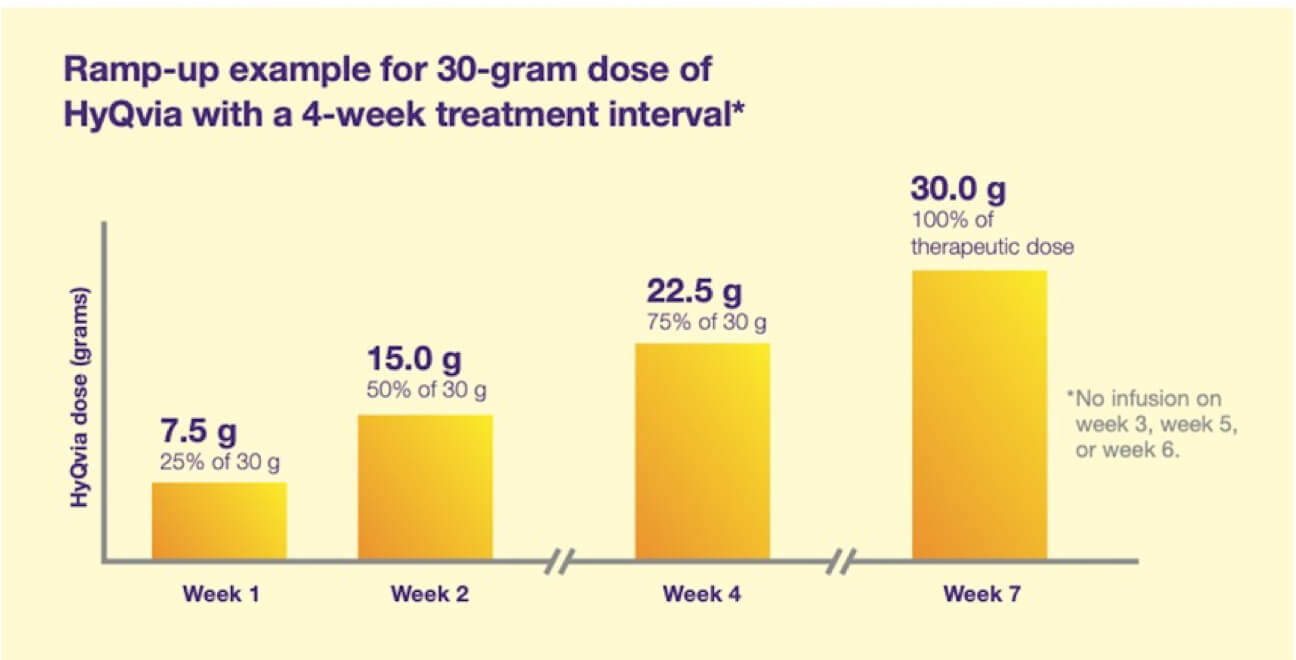
How to ramp up HyQvia
There is a ramp-up period for HyQvia which allows patients to become familiar with the volumes required for a full 3- or 4-week treatment.1
Initial treatment and HyQvia ramp-up schedule
Treatment with HyQvia is initiated by gradually increasing the dose and decreasing the frequency from a 1-week dose to a 3- or 4-week dose.
Dosage during this ramp-up period is calculated by dividing the total 3- or 4-week therapeutic dose as shown in the graph.
The first dose of HyQvia should be given approximately 1 week after the last infusion of the previous treatment.
To access patient resources, visit Access & Support.
For more comprehensive information on HyQvia dosing, see Full Prescribing Information
Infusion experience
Over the course of the efficacy and extension trials of HyQvia in ~3.5 years (2959 infusions), there were no clinically observable changes in the skin or subcutaneous tissue.1
Individual results may vary.
Pre-Infusion

Patient weight: 168 lb
Volume: 500 mL
Number of Infusion sites: 1
Post-Infusion
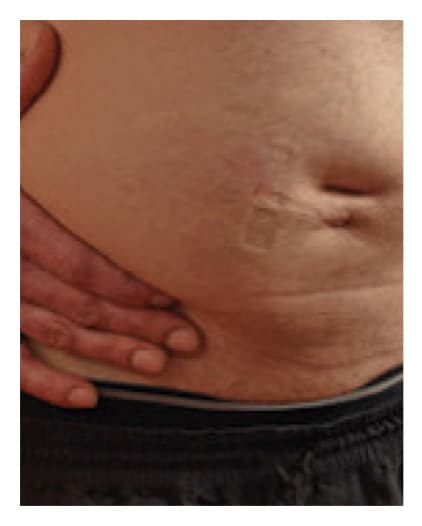
Results in a diffuse, temporary, localized swelling
24 Hours Post-Infusion
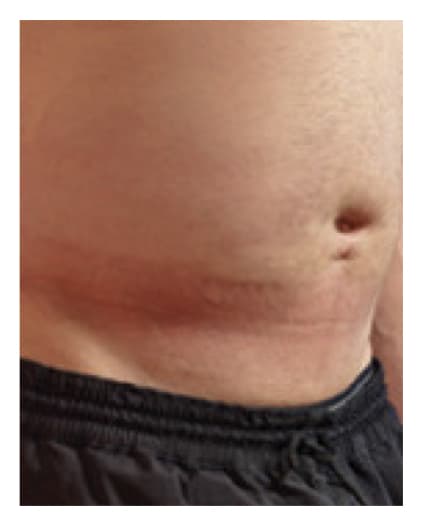
Infusion site swelling generally resolved within 1 to 3 days
Pre-Infusion
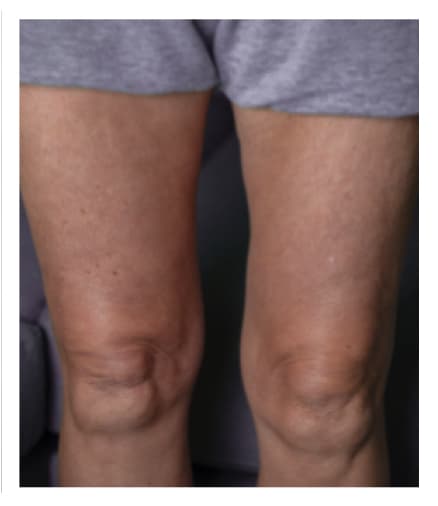
Patient weight: 162 lb
Volume: 300 mL
Number of Infusion sites: 2
Post-Infusion

Side effects include acute tenderness
24 Hours Post-Infusion
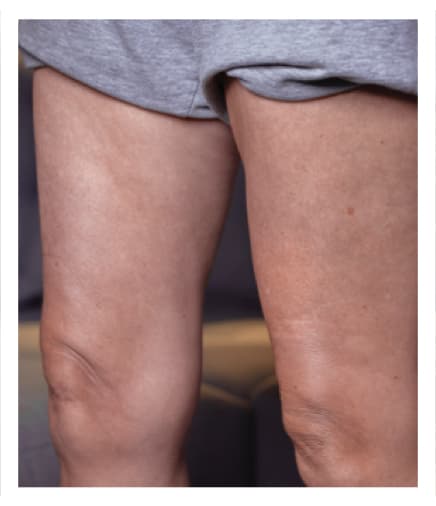
Infusion site swelling generally resolved within 1 to 3 days
Infusion time estimator
See how HyQvia patients could spend less than half the time infusing compared to conventional IVIG1
How long do they spend infusing?
Monthly dose (in grams)
monthly
1 site
2 sites
*Every 3 or 4 weeks.
Flexible administration options1
You can work with your patients to determine:
- Either 1 or 2 infusion sites
- A second site can be used at the discretion of the physician and patient based on tolerability and total volume
- Infusion site location in either the abdomen or the thighs
- Every 3- or 4-week treatment intervals
- Infuse at home, after adequate training, or in-center
Infusion options for your patients
To better meet the needs of your patients, there are 2 different methods available to prepare and infuse HyQvia.1 Depending on the specialty pharmacy, patients will receive one of the following pump options:
Peristaltic pump and pooling bag
Hy component is infused by manual syringe push. IG component is infused by the peristaltic pump.
Download our brochure on how to infuse HyQvia with a peristaltic pump.
Watch our peristaltic pump infusion video:
- 0:00 Get familiar with hy5
- 3:35 hy1: Get ready
- 4:31 hy2: Prepare the hyaluronidase, or Hy
- 6:42 hy3: Prepare the immune globulin, or IG
- 10:37 hy4: Infuse
- 15:30 hy5: Finsh up
Syringe driver pump
Hy component is infused by manual syringe push or the syringe driver pump. IG component is infused by the syringe driver pump.
A range of syringe driver pumps meet the criteria to administer HyQvia.
Download our brochure on how to infuse HyQvia with a syringe driver pump.
Reference
- HyQvia. Prescribing information. Takeda Pharmaceuticals U.S.A., Inc.; 2024.